
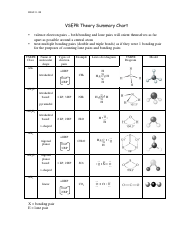
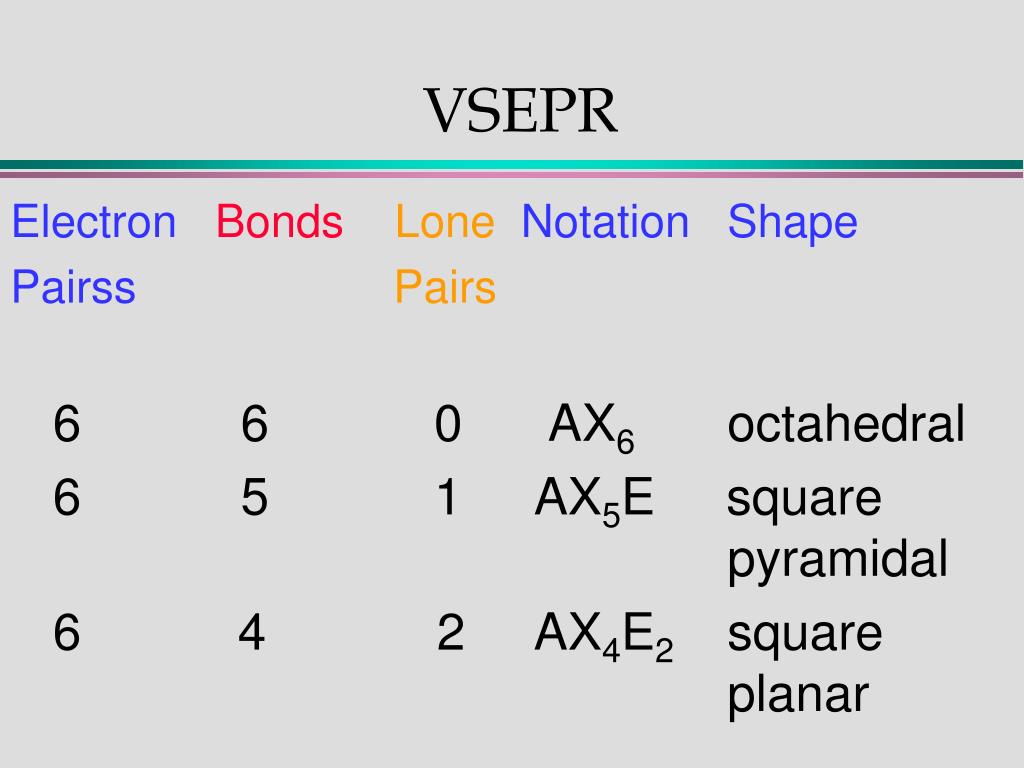
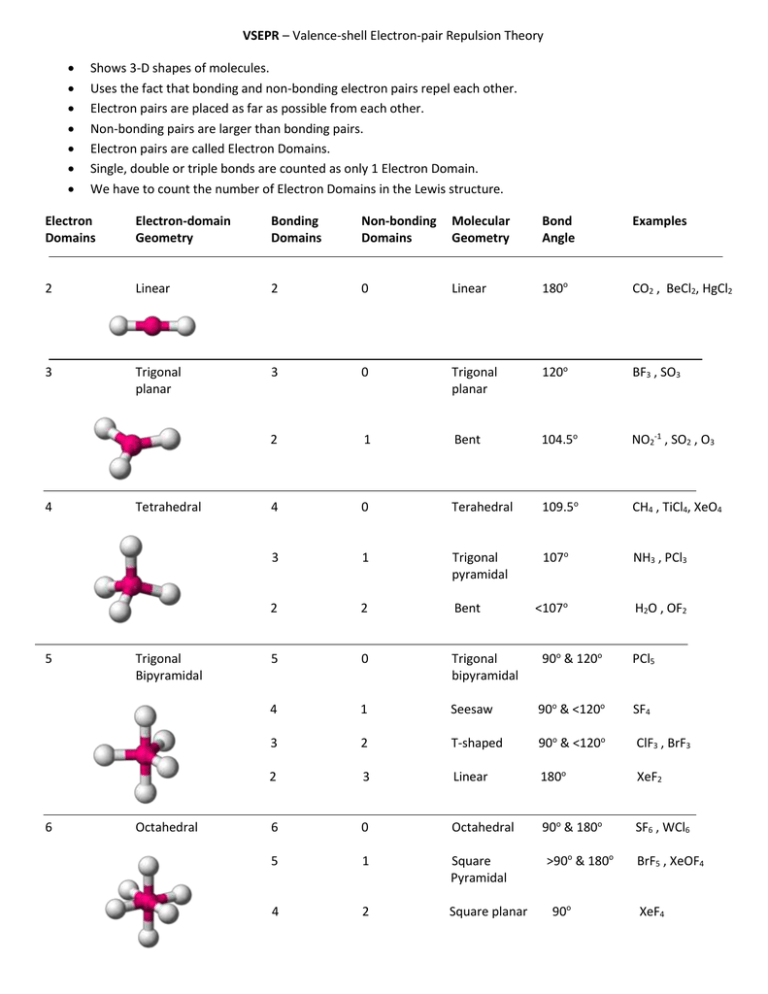
Lewis structures highlight the presence of bonded electrons and electron lone pairs. This can skew the shape to different conformations. Often in molecular geometries, the shape will be different, especially the values of the angles, as lone pairs will exert greater repulsion than bonded electrons. Secondly, we have to consider that a lone pair of electrons will have a greater repulsion than a bonded pair of electrons. Firstly, a double bond will behave differently to a single bond, yet the theory will regard it as a single electron domain. Key assumptions we have to consider are the differences in the bonds, and species of electron domains. This theory focuses on these electron repulsions since, according to VSEPR, the geometry that the molecule will adopt is the one in which the electron groups have the maximum separation possible from each other. VSEPR theory is based on the very straightforward idea that electron groups - which can either consist of lone pairs of electrons, single bonds, multiple bonds, or unpaired electrons - repel each other. It is necessary to understand the basic principles the theory is based on, such as the principles of electron pair behaviour within a molecule, most commonly represented by Lewis structures. It can also help to deduce the shape of molecules from 2D representations to 3D structures. The VSEPR theory can explain why certain molecules are shaped the way they are in 3D. VSEPR theory defines the three-dimensional shape of molecules based on the repulsion of electron pairs and the presence of bonds.
Variable Oxidation State of Transition Elements.Transition Metal Ions in Aqueous Solution.


 0 kommentar(er)
0 kommentar(er)
